

DSP-107
DSP-107
BEST IN CLASS
CD47 TARGETING
CD47 TARGETING
BEST IN CLASS
CD47 TARGETING
CD47 TARGETING
Novel functional configuration
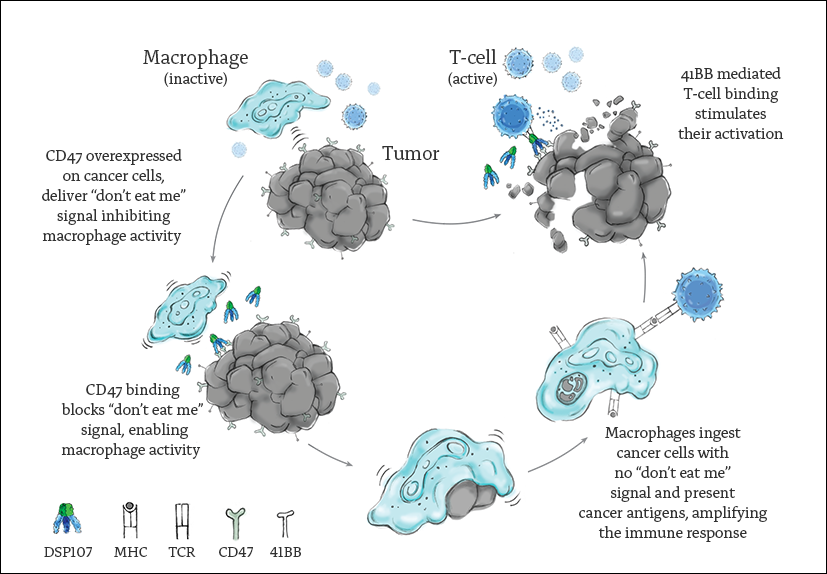
DSP107’s (Dual Signaling Protein) unique design facilitates a targeted activation of both innate and adaptive immunity in the tumor microenvironment (TME).
DSP107’s functional arms are composed of the extracellular domains of 4-1BB Ligand (4-1BBL) and SIRPα.
The dual functional arms trigger a local, synergistic immune response by binding CD47 over-expressed on cancer cells, disabling their “don’t eat me” signal, followed by binding to 4-1BB receptors expressed on activated, tumor-reactive T-cells stimulating their proliferation and activation. The trimeric binding of DSP107 to CD47 enables cross presentation of 4-1BBL for conditional, tumor-localized, 4-1BB receptor activation on tumor reactive T-cells.
DSP107’s functional arms are composed of the extracellular domains of 4-1BB Ligand (4-1BBL) and SIRPα.
The dual functional arms trigger a local, synergistic immune response by binding CD47 over-expressed on cancer cells, disabling their “don’t eat me” signal, followed by binding to 4-1BB receptors expressed on activated, tumor-reactive T-cells stimulating their proliferation and activation. The trimeric binding of DSP107 to CD47 enables cross presentation of 4-1BBL for conditional, tumor-localized, 4-1BB receptor activation on tumor reactive T-cells.
UNIQUE ADVANTAGES
Activating both innate
and adaptive immunity
Favorable safety – no binding to RBCs
No off-target toxicities - Localized, conditional 41BB activation
Opportunity for solid tumors – high unmet need
The science of conditional trimeric binding
CD47 is a transmembrane protein that functions as an anti-phagocytic “don’t eat me” signal for healthy cells. CD47 binds SIRPα, an inhibitory transmembrane protein that is expressed on macrophages and other myeloid cells, resulting in the inhibition of phagocytosis. By virtue of CD47 overexpression, cancer cells are able to evade innate immune system detection by macrophages and dendritic cells (DCs), and subsequent antigen presentation to T-cells thereby limiting anti-tumor immune response.
Current approaches to blocking CD47 have shown very promising efficacy in multiple cancer indications establishing CD47 as an important target. However, they also encountered some limitations that challenged their ability to maximize the full potential of this therapeutic avenue. Blocking with monoclonal antibodies and Fc-fusion proteins resulted in on target/off tumor effects and hematological toxicities. In addition, CD47 agents that bind red blood cells are hampered by antigen sink requiring high and frequent dosing to achieve the necessary exposure level.
DSP107 overcomes these limitations by not presenting an Fc region, not binding to red blood cells and with its SIRPα-41BBL trimeric arms it ensures selective binding to tumors with densely expressed CD47 and tumor localized T cell activation.
Current approaches to blocking CD47 have shown very promising efficacy in multiple cancer indications establishing CD47 as an important target. However, they also encountered some limitations that challenged their ability to maximize the full potential of this therapeutic avenue. Blocking with monoclonal antibodies and Fc-fusion proteins resulted in on target/off tumor effects and hematological toxicities. In addition, CD47 agents that bind red blood cells are hampered by antigen sink requiring high and frequent dosing to achieve the necessary exposure level.
DSP107 overcomes these limitations by not presenting an Fc region, not binding to red blood cells and with its SIRPα-41BBL trimeric arms it ensures selective binding to tumors with densely expressed CD47 and tumor localized T cell activation.
4-1BB is a member of the tumor necrosis factor receptor (TNFR) superfamily and has diverse functions in the immune system, one of which is providing co-stimulatory signals during an immune response. Co-stimulatory TNFR signaling is pivotal for effective T-cell immunity and is therefore attractive as a therapeutic strategy to induce or restore effective antitumor immunity.
A host of preclinical data have shown that activation of antitumor T-cell immune responses, using agonistic antibodies that target their respective co-stimulatory receptors, triggers potent antitumor immunity.
Targeting 4-1BB emerged as a promising therapeutic strategy for cancer immunotherapy as it is expressed on diverse T-cell populations upon activation, including cytotoxic and helper T-cells, natural killer (NK) cells and natural killer T-cells (NKT). The only known ligand for 4-1BB is 4-1BBL, expressed by activated Antigen Presenting Cells (APCs), including B-cells, dendritic cells (DCs) and macrophages. Upon ligation and crosslinking, 4-1BB is stimulated and increases signaling through the T-cell receptor (TCR) and amplifies the cytotoxicity of CD8+ T-cells. Similarly, in NK cells, 4-1BB stimulation enhances proliferation, IFN-γ production and cytolytic activity. 4-1BB ligation increases the secretion of perforin and granzyme and the activation of the Fas ligand effector system by both CD8+ T- and NK cells.
4-1BB signaling is only activated by membrane-expressed 4-1BBL. Soluble 4-1BBL binds 4-1BB but lacks activity.
Agonistic monoclonal antibodies targeting 4-1BB require FcR-crosslinking in order to effectively activate their target. This FcR-mediated constitutive cross-linking results in uncontrolled, non-localized activity leading to hepato-toxicity.
Targeted delivery of 4-1BBL can be exploited to achieve a locally active 4-1BB agonist. This feature is utilized by DSP107 to trigger tumor-localized 4-1BB activation that requires CD47 binding. DSP107 is inactive 'en route', and the cross presentation of trimeric 4-1BBL, following DSP107 binding to the cancer, imitates naturally occurring, membrane-bound homo-trimer 4-1BBL forms. Upon engagement with their receptor, 4-1BB trimerizes, resulting in a strong co-stimulatory signal.
A host of preclinical data have shown that activation of antitumor T-cell immune responses, using agonistic antibodies that target their respective co-stimulatory receptors, triggers potent antitumor immunity.
Targeting 4-1BB emerged as a promising therapeutic strategy for cancer immunotherapy as it is expressed on diverse T-cell populations upon activation, including cytotoxic and helper T-cells, natural killer (NK) cells and natural killer T-cells (NKT). The only known ligand for 4-1BB is 4-1BBL, expressed by activated Antigen Presenting Cells (APCs), including B-cells, dendritic cells (DCs) and macrophages. Upon ligation and crosslinking, 4-1BB is stimulated and increases signaling through the T-cell receptor (TCR) and amplifies the cytotoxicity of CD8+ T-cells. Similarly, in NK cells, 4-1BB stimulation enhances proliferation, IFN-γ production and cytolytic activity. 4-1BB ligation increases the secretion of perforin and granzyme and the activation of the Fas ligand effector system by both CD8+ T- and NK cells.
4-1BB signaling is only activated by membrane-expressed 4-1BBL. Soluble 4-1BBL binds 4-1BB but lacks activity.
Agonistic monoclonal antibodies targeting 4-1BB require FcR-crosslinking in order to effectively activate their target. This FcR-mediated constitutive cross-linking results in uncontrolled, non-localized activity leading to hepato-toxicity.
Targeted delivery of 4-1BBL can be exploited to achieve a locally active 4-1BB agonist. This feature is utilized by DSP107 to trigger tumor-localized 4-1BB activation that requires CD47 binding. DSP107 is inactive 'en route', and the cross presentation of trimeric 4-1BBL, following DSP107 binding to the cancer, imitates naturally occurring, membrane-bound homo-trimer 4-1BBL forms. Upon engagement with their receptor, 4-1BB trimerizes, resulting in a strong co-stimulatory signal.
Get in Touch
KAHR Dam HaMacbim St 28, POB 9 Modi'in Makabim-Re'ut 7178594, Israel T. +972.73.7969196 [email protected]