

DSP-FC 216
DSP-FC 216
Synergistic HLA-G and
CD47 blockade
CD47 blockade
Synergistic HLA-G and
CD47 blockade
CD47 blockade
Novel functional configuration
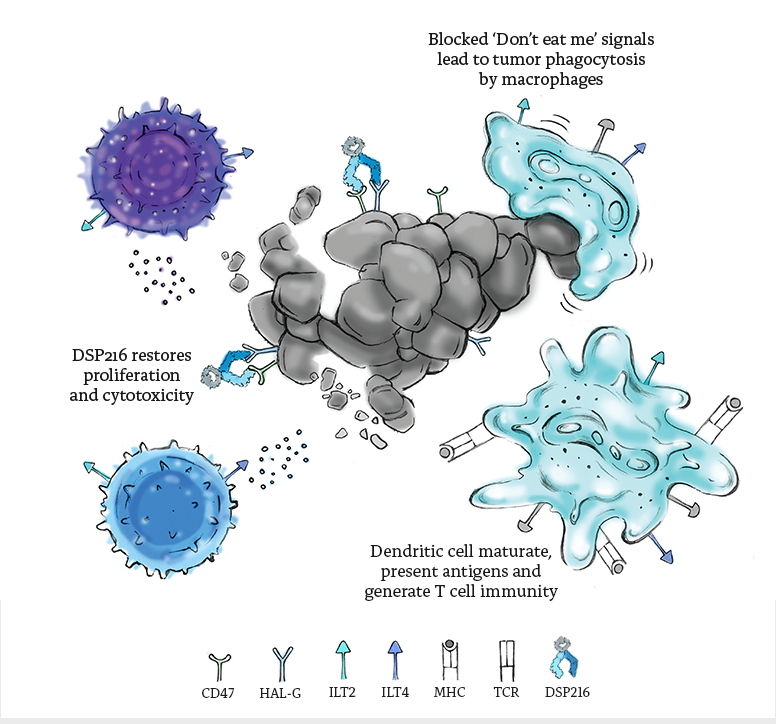
DSP-FC 216, with its SIRPα and LILRB2 arms directly targets both HLA-G expressed exclusively in the TME and CD47 overexpressed on the same cells, ensuring strict tumor targeting through dual checkpoint binding. HLA-G blockade interferes with both LILRB1 and LILRB2 binding to avoid redundancy compensation and activates the innate and adaptive immune systems while CD47 blockade simultaneously triggers phagocytosis of tumor cells.
UNIQUE ADVANTAGES

Multiple inhibition of
checkpoint pathways
Activating innate and
adaptive immunity
Favorable safety –
no binding to RBCs
Overcome LILRBs’
redundancy compensation
DUAL CHECKPOINT SCIENCE
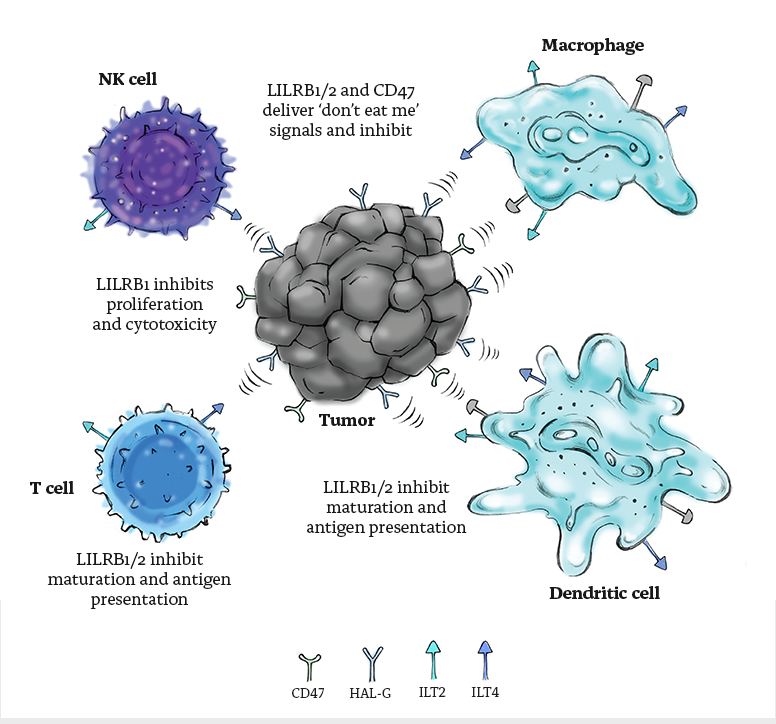
LILRB1 (ILT2) and LILRB2 (ILT4) are members of the leukocyte Ig-like receptor (LIR) family. LILRB1 is expressed on monocytes, DCs, B cells, and subsets of NK and T cells, whereas LILRB2 is almost exclusively expressed on myeloid cells (monocytes, DCs, macrophages and neutrophils).
HLA-G, the main ligand for LILRB1 and LILRB2, is a MHC class I molecule selectively expressed on placenta at the fetal-maternal interface, to prevent the mother’s immune system from destroying the fetus. By over-expressing HLA-G, tumor cells use the same escape mechanism to evade immune surveillance. HLA-G expression level is associated with cancer immune evasion, disease progression and poor prognosis. HLA-G serves as a broad-range immune checkpoint inhibitor (ICI) and by its interaction with LILRB1 and LILRB2 it inhibits all immune response players (B, T, and NK cells, monocytes/DC and, neutrophils) and recruits suppressive APC and Treg cells to the TME inducing an immunosuppressive microenvironment for tumors.
HLA-G, the main ligand for LILRB1 and LILRB2, is a MHC class I molecule selectively expressed on placenta at the fetal-maternal interface, to prevent the mother’s immune system from destroying the fetus. By over-expressing HLA-G, tumor cells use the same escape mechanism to evade immune surveillance. HLA-G expression level is associated with cancer immune evasion, disease progression and poor prognosis. HLA-G serves as a broad-range immune checkpoint inhibitor (ICI) and by its interaction with LILRB1 and LILRB2 it inhibits all immune response players (B, T, and NK cells, monocytes/DC and, neutrophils) and recruits suppressive APC and Treg cells to the TME inducing an immunosuppressive microenvironment for tumors.
CD47 is a transmembrane protein that functions as an anti-phagocytic “don’t eat me” signal for healthy cells. CD47 binds SIRPα, an inhibitory transmembrane protein that is expressed on macrophages and other myeloid cells, resulting in the inhibition of phagocytosis. By virtue of CD47 overexpression, cancer cells are able to evade innate immune system detection by macrophages and dendritic cells (DCs), and subsequent antigen presentation to T-cells thereby limiting anti-tumor immune response.
Get in Touch
KAHR Dam HaMacbim St 28, POB 9 Modi'in Makabim-Re'ut 7178594, Israel T. +972.73.7969196 [email protected]